- UPSC LABS
- March 03, 2025
- 6:35 pm
- Ratings: ⭐⭐⭐⭐⭐
CRISPR-Cas9: Mechanism, Ethical Considerations, and Applications
The advent of CRISPR-Cas9 technology has revolutionized the field of genetic engineering, offering unprecedented precision, efficiency, and versatility in gene editing. Derived from a bacterial immune system, CRISPR-Cas9 allows scientists to make targeted modifications to the DNA of living organisms, opening up new possibilities in medicine, agriculture, and biotechnology. This article provides a comprehensive overview of the mechanism of CRISPR-Cas9, its ethical considerations, and its applications in gene editing, with a special focus on its relevance to India.
The discovery of CRISPR-Cas9 has been hailed as one of the most significant scientific breakthroughs of the 21st century. Its potential to edit genes with high precision has far-reaching implications, from curing genetic diseases to creating climate-resilient crops. However, the technology also raises profound ethical, social, and regulatory questions that must be addressed to ensure its responsible use.
Table of Contents
Discovery and Origin
The CRISPR-Cas9 system was first discovered in bacteria and archaea, where it functions as an adaptive immune system to defend against viral infections. The term CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats, which are sequences of DNA that bacteria use to “remember” and recognize viral invaders. The Cas9 protein, an enzyme, acts as molecular scissors that cut the DNA at specific locations.
The discovery of CRISPR-Cas9 was a result of decades of research into bacterial immune systems. In 1987, scientists observed unusual repetitive sequences in the DNA of E. coli, but their function remained unknown for years. It was not until the early 2000s that researchers realized these sequences were part of a bacterial defense mechanism. In 2012, Jennifer Doudna and Emmanuelle Charpentier demonstrated that CRISPR-Cas9 could be programmed to cut specific DNA sequences in a test tube, paving the way for its use as a gene-editing tool.
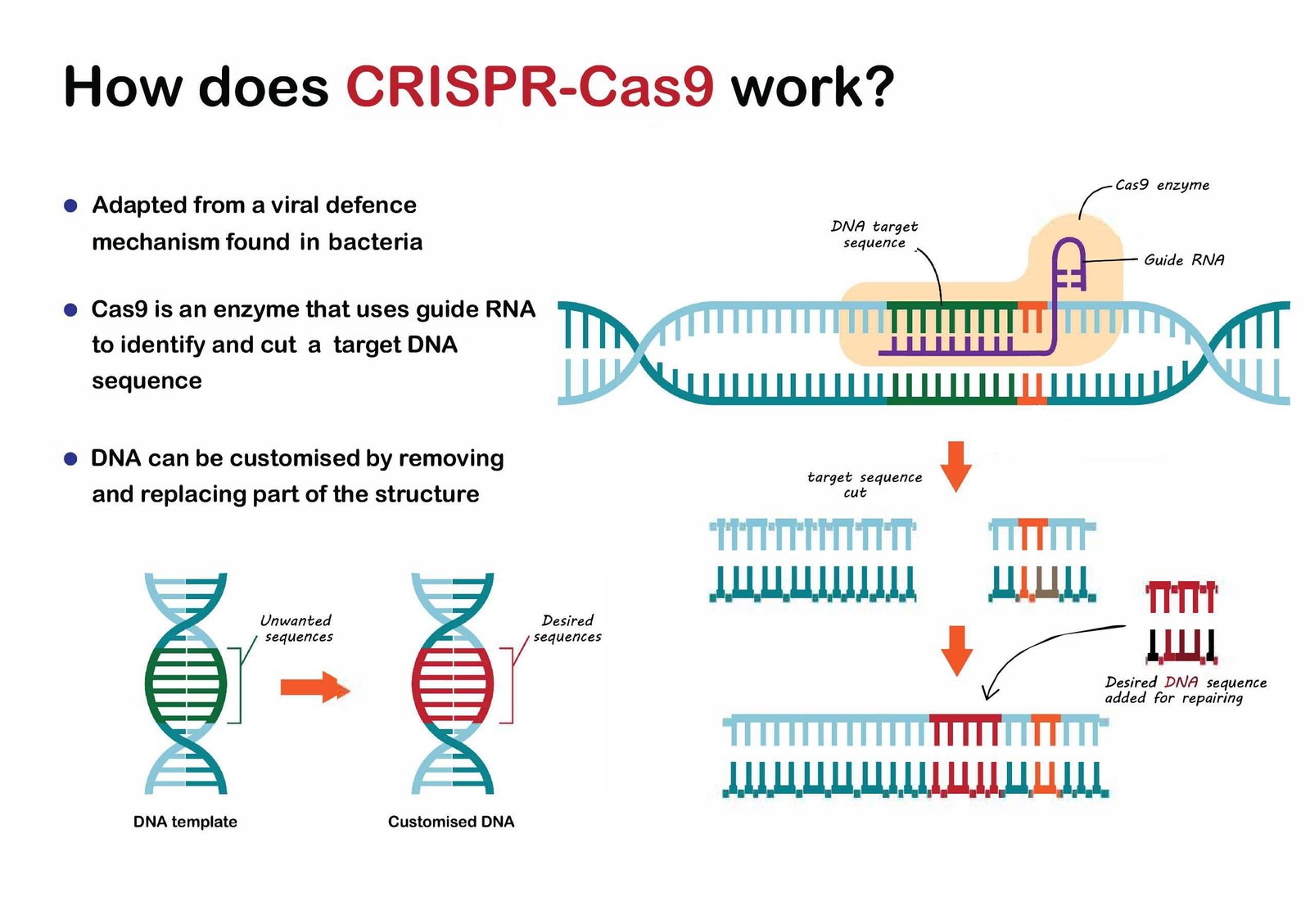
How CRISPR-Cas9 Works
The CRISPR-Cas9 system consists of two key components:
Guide RNA (gRNA): A short RNA sequence that is complementary to the target DNA sequence. The gRNA directs the Cas9 protein to the precise location in the genome that needs to be edited.
Cas9 Protein: An enzyme that cuts the DNA at the location specified by the gRNA. Once the DNA is cut, the cell’s natural repair mechanisms take over, either introducing mutations or inserting new genetic material.
The process involves three main steps:
Target Identification: The gRNA binds to the target DNA sequence through complementary base pairing.
DNA Cleavage: The Cas9 protein cuts both strands of the DNA at the target site, creating a double-strand break.
DNA Repair: The cell repairs the break using one of two mechanisms:
Non-Homologous End Joining (NHEJ): This error-prone process often introduces small insertions or deletions, disrupting the gene.
Homology-Directed Repair (HDR): This precise mechanism uses a DNA template to introduce specific changes or insert new genetic material.
The simplicity and efficiency of CRISPR-Cas9 have made it a powerful tool for genetic engineering. Unlike earlier technologies, which required complex protein engineering, CRISPR-Cas9 relies on RNA-DNA interactions, making it easier to design and implement.
Advantages of CRISPR-Cas9
CRISPR-Cas9 offers several advantages over earlier gene-editing technologies, such as Zinc Finger Nucleases (ZFNs) and Transcription Activator-Like Effector Nucleases (TALENs):
Precision: The ability to target specific DNA sequences with high accuracy.
Efficiency: Faster and more cost-effective than previous methods.
Versatility: Applicable to a wide range of organisms, from bacteria to humans.
These advantages have made CRISPR-Cas9 the tool of choice for researchers worldwide, leading to rapid advancements in fields such as medicine, agriculture, and biotechnology.
Applications of CRISPR-Cas9 in Medicine
CRISPR-Cas9 has transformative potential in medicine, offering new ways to treat and prevent diseases.
Genetic Disorders
CRISPR can be used to correct mutations responsible for genetic disorders such as sickle cell anemia, cystic fibrosis, and Duchenne muscular dystrophy. Clinical trials are already underway to test CRISPR-based therapies for these conditions. For example, CRISPR Therapeutics and Vertex Pharmaceuticals have developed a CRISPR-based treatment for sickle cell anemia, which has shown promising results in early trials.
Cancer
CRISPR is being explored as a tool for cancer immunotherapy. By editing the genes of immune cells, scientists can enhance their ability to recognize and destroy cancer cells. For example, CAR-T cell therapy, which uses CRISPR to engineer immune cells, has shown promising results in treating certain types of leukemia.
Infectious Diseases
CRISPR can also be used to combat infectious diseases. Researchers are developing CRISPR-based diagnostics to detect pathogens such as HIV, Zika virus, and SARS-CoV-2 with high sensitivity and specificity. Additionally, CRISPR is being explored as a tool to disable viral genomes, potentially leading to new treatments for viral infections.
Applications of CRISPR-Cas9 in Agriculture
CRISPR-Cas9 is revolutionizing agriculture by enabling the development of crops with improved traits.
Disease Resistance
Scientists are using CRISPR to create crops that are resistant to diseases, pests, and environmental stressors. For example, CRISPR-edited rice varieties have been developed to withstand bacterial blight, a major threat to global food security.
Nutritional Enhancement
CRISPR can be used to enhance the nutritional content of crops. For instance, researchers have used CRISPR to increase the vitamin A content in bananas, addressing malnutrition in developing countries.
Climate Resilience
CRISPR is being used to develop crops that are more resilient to climate change, such as drought-tolerant maize and heat-resistant wheat. These innovations could play a critical role in ensuring food security in the face of global warming.
Ethical Considerations
Potential for Misuse
The power of CRISPR-Cas9 raises significant ethical concerns, particularly regarding its potential for misuse. One of the most controversial applications is germline editing, which involves making heritable changes to the DNA of embryos. While this could eliminate genetic diseases, it also raises the possibility of designer babies, where parents select traits such as intelligence, appearance, or athletic ability. Such practices could exacerbate social inequalities and lead to a new form of eugenics.
The case of He Jiankui, a Chinese scientist who claimed to have created the world’s first CRISPR-edited babies in 2018, highlights the risks of unregulated germline editing. His experiment, which aimed to make the babies resistant to HIV, was widely condemned for its lack of transparency, ethical oversight, and potential long-term consequences.
Equity and Access
Another ethical issue is the unequal access to CRISPR-based therapies. Advanced treatments are often expensive and may only be available to wealthy individuals or nations, widening the gap between the rich and the poor. Ensuring equitable access to gene-editing technologies is a major challenge for policymakers and healthcare systems worldwide.
For example, CRISPR-based therapies for diseases like sickle cell anemia and beta-thalassemia have shown promising results in clinical trials, but their high cost could limit their availability to patients in low- and middle-income countries. Addressing this issue will require international cooperation, innovative financing mechanisms, and policies that prioritize public health over profit.
Environmental Impact
The use of CRISPR-Cas9 in agriculture and environmental management also raises ethical questions. For example, gene drives—a technology that uses CRISPR to spread specific genes through wild populations—could be used to eradicate disease-carrying insects or invasive species. However, this could have unintended ecological consequences, such as disrupting ecosystems or creating new imbalances.
The release of gene-drive organisms into the wild is a contentious issue, with some scientists advocating for caution and others pushing for rapid deployment to address urgent problems like malaria. Balancing the potential benefits of gene drives with their risks will require robust regulatory frameworks, public engagement, and interdisciplinary collaboration.
Regulatory Challenges
The rapid development of CRISPR technology has outpaced the establishment of regulatory frameworks. Governments and international organizations face the challenge of balancing innovation with safety, ensuring that gene-editing research is conducted responsibly and transparently.
In the United States, the National Institutes of Health (NIH) and the Food and Drug Administration (FDA) have established guidelines for CRISPR research, but these are not legally binding. In Europe, the European Court of Justice has ruled that CRISPR-edited organisms should be regulated as genetically modified organisms (GMOs), subjecting them to strict oversight. These differing approaches highlight the need for global harmonization of CRISPR regulations.
CRISPR-Cas9 in India: Opportunities and Challenges
Research and Development
India has emerged as a significant player in CRISPR research, with several institutions and startups actively working on gene-editing technologies. The Indian Institute of Science (IISc), Tata Institute of Fundamental Research (TIFR), and Centre for Cellular and Molecular Biology (CCMB) are among the leading centers for CRISPR research in the country.
Indian researchers have made notable contributions to the field, such as developing CRISPR-based tools for studying gene function and creating disease-resistant crops. However, funding constraints and infrastructure limitations remain significant challenges for CRISPR research in India.
Agricultural Applications
India’s agricultural sector stands to benefit greatly from CRISPR technology. With a large population dependent on farming, the development of disease-resistant, drought-tolerant, and nutritionally enhanced crops could address critical challenges such as food security and farmer livelihoods. For example, CRISPR-edited mustard and rice varieties are being developed to improve yield and resilience.
However, the adoption of CRISPR-edited crops in India faces regulatory and public acceptance hurdles. The Genetic Engineering Appraisal Committee (GEAC), which oversees genetically modified organisms (GMOs), has yet to establish clear guidelines for CRISPR-edited crops. Public skepticism about genetic engineering, fueled by controversies over Bt cotton and Bt brinjal, could also hinder the acceptance of CRISPR-edited crops.
Healthcare Innovations
CRISPR-based therapies have the potential to address some of India’s most pressing healthcare challenges, such as genetic disorders, infectious diseases, and cancer. However, the high cost of these therapies poses a significant barrier to their widespread adoption.
India’s biotechnology industry is actively exploring CRISPR-based solutions for diseases like thalassemia and sickle cell anemia, which are prevalent in the country. Collaborations between academia, industry, and government will be crucial for translating CRISPR research into affordable and accessible therapies.
Regulatory Framework
India is in the process of developing a regulatory framework for gene-editing technologies. The Department of Biotechnology (DBT) and the Genetic Engineering Appraisal Committee (GEAC) are responsible for overseeing CRISPR research and applications. Ensuring that regulations are both robust and flexible will be critical to fostering innovation while addressing ethical and safety concerns.
Ethical and Social Implications
India’s diverse socio-economic landscape presents unique ethical challenges for CRISPR technology. Issues such as equitable access, informed consent, and cultural sensitivities must be carefully considered to ensure that the benefits of gene editing are shared by all sections of society.
Public engagement and education will be essential for building trust and addressing misconceptions about CRISPR technology. Policymakers must also consider the potential impact of CRISPR on marginalized communities, ensuring that the technology does not exacerbate existing inequalities.
Conclusion
CRISPR-Cas9 represents a groundbreaking advancement in genetic engineering, with far-reaching implications for medicine, agriculture, and biotechnology. Its ability to make precise, targeted changes to DNA has opened up new possibilities for treating diseases, improving crops, and addressing environmental challenges. However, the technology also raises significant ethical and regulatory questions that must be addressed to ensure its responsible and equitable use.
For India, CRISPR offers immense opportunities to address critical challenges in healthcare and agriculture. However, realizing this potential will require a concerted effort to build research capacity, develop appropriate regulatory frameworks, and address ethical and social concerns. As CRISPR technology continues to evolve, it will be essential to strike a balance between innovation and responsibility, ensuring that its benefits are shared by all.
