- UPSC LABS
- March 03, 2025
- 6:35 pm
- Ratings: ⭐⭐⭐⭐⭐
Gene Therapy: Types, Mechanisms, and Advancements
Gene therapy represents one of the most revolutionary advancements in modern medicine, offering the potential to treat, cure, or prevent diseases by modifying the genetic material of living cells. This innovative approach targets the root cause of genetic disorders, providing hope for conditions that were once considered incurable. From rare monogenic diseases to complex multifactorial disorders, gene therapy has the potential to transform healthcare.
Table of Contents
What is Gene Therapy?
Gene therapy is a medical technique that involves the introduction, alteration, or removal of genetic material within a patient’s cells to treat or prevent disease. The primary goal is to correct defective genes responsible for disease development or to introduce new genes that can help fight disease. This approach can be applied to both inherited genetic disorders, such as cystic fibrosis and sickle cell anemia, and acquired diseases, such as cancer and viral infections.
The concept of gene therapy emerged in the 1970s, but it was not until the 1990s that the first clinical trials were conducted. Since then, the field has evolved significantly, with several gene therapies now approved for clinical use. The success of gene therapy depends on the efficient delivery of therapeutic genes to target cells, their stable integration into the genome, and the precise regulation of gene expression.
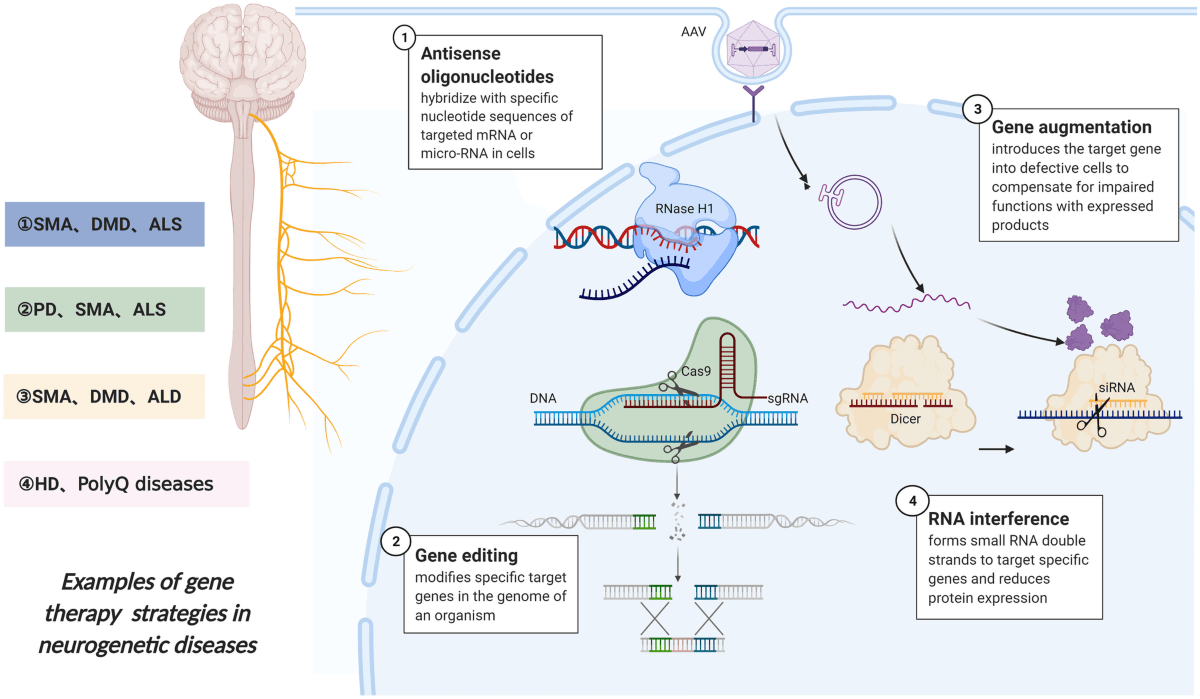
Types of Gene Therapy
Gene therapy can be broadly classified into two categories: somatic gene therapy and germline gene therapy.
Somatic Gene Therapy
Somatic gene therapy involves the modification of genes in somatic (non-reproductive) cells. The changes made are not passed on to future generations, making this approach ethically less controversial. Somatic gene therapy is further divided into in vivo and ex vivo approaches.
In Vivo Gene Therapy: In this approach, therapeutic genes are directly delivered into the patient’s body using viral or non-viral vectors. The vectors transport the genes to the target cells, where they are expressed to produce the desired therapeutic effect. Examples include the treatment of inherited retinal diseases using adeno-associated virus (AAV) vectors.
Ex Vivo Gene Therapy: This approach involves extracting cells from the patient, genetically modifying them in the laboratory, and then reintroducing them into the patient’s body. This method is commonly used in the treatment of blood disorders, such as beta-thalassemia and severe combined immunodeficiency (SCID).
Germline Gene Therapy
Germline gene therapy involves the modification of genes in reproductive cells (sperm, eggs, or embryos). The changes made are heritable and can be passed on to future generations. While this approach has the potential to eradicate genetic diseases at their source, it raises significant ethical concerns and is currently prohibited in most countries.

Mechanisms of Gene Therapy
The success of gene therapy depends on the efficient delivery of therapeutic genes to target cells and their stable integration into the genome. Several mechanisms are employed to achieve this, including the use of viral and non-viral vectors, gene editing technologies, and RNA-based therapies.
Viral Vectors
Viruses have evolved to efficiently deliver their genetic material into host cells, making them ideal vectors for gene therapy. The most commonly used viral vectors include:
Adenoviruses: These vectors can carry large genes and infect a wide range of cell types. However, they often trigger an immune response and do not integrate into the host genome, resulting in transient gene expression.
Retroviruses: Retroviral vectors integrate into the host genome, ensuring stable and long-term gene expression. However, their integration is random, which can lead to insertional mutagenesis and the risk of cancer.
Lentiviruses: A subclass of retroviruses, lentiviruses can infect both dividing and non-dividing cells. They are widely used in ex vivo gene therapy for blood disorders.
Adeno-Associated Viruses (AAV): AAV vectors are small, non-pathogenic, and can infect a wide range of cell types. They provide long-term gene expression without integrating into the host genome, making them safer than retroviruses.
Non-Viral Vectors
Non-viral vectors offer several advantages, including lower immunogenicity, ease of production, and the ability to carry larger genetic payloads. However, they are generally less efficient than viral vectors. Common non-viral delivery methods include:
Naked DNA: Direct injection of plasmid DNA into target tissues. This method is simple but inefficient and results in transient gene expression.
Liposomes: Lipid-based nanoparticles that encapsulate DNA and facilitate its delivery into cells. Liposomes are widely used in cancer gene therapy.
Electroporation: The application of an electric field to create temporary pores in the cell membrane, allowing DNA to enter the cell. This method is commonly used in ex vivo gene therapy.
Gene Editing Technologies
Gene editing technologies, such as CRISPR-Cas9, have revolutionized the field of gene therapy by enabling precise modifications to the genome. CRISPR-Cas9 uses a guide RNA to target specific DNA sequences, where the Cas9 enzyme introduces double-strand breaks. These breaks can be repaired by the cell’s natural repair mechanisms, allowing for the insertion, deletion, or correction of genetic material.
RNA-Based Therapies
RNA-based therapies involve the use of RNA molecules to modulate gene expression. Examples include:
Small Interfering RNA (siRNA): siRNA molecules can silence specific genes by degrading their mRNA, preventing the production of harmful proteins.
Antisense Oligonucleotides (ASOs): ASOs are short, single-stranded DNA molecules that bind to mRNA and prevent its translation into protein.

Advancements in Gene Therapy
The field of gene therapy has witnessed remarkable advancements over the past few decades, leading to the approval of several therapies for clinical use. Some of the most significant advancements include:
Approved Gene Therapies
Luxturna: Approved in 2017, Luxturna is a gene therapy for the treatment of inherited retinal diseases caused by mutations in the RPE65 gene. It uses an AAV vector to deliver a functional copy of the RPE65 gene to retinal cells, restoring vision in patients.
Zolgensma: Approved in 2019, Zolgensma is a gene therapy for spinal muscular atrophy (SMA), a severe neuromuscular disorder. It uses an AAV vector to deliver a functional copy of the SMN1 gene to motor neurons, improving muscle function and survival in patients.
Kymriah and Yescarta: These are chimeric antigen receptor (CAR) T-cell therapies approved for the treatment of certain types of blood cancer. They involve the genetic modification of a patient’s T cells to express a receptor that targets cancer cells, leading to their destruction.
Emerging Technologies
Base Editing: A newer gene editing technology that allows for the precise conversion of one DNA base pair into another without causing double-strand breaks. This approach reduces the risk of unintended mutations and is being explored for the treatment of genetic disorders caused by single-nucleotide mutations.
Prime Editing: An advanced gene editing technology that can insert, delete, or replace DNA sequences with high precision. Prime editing offers greater flexibility and accuracy than CRISPR-Cas9 and has the potential to correct a wide range of genetic mutations.
In Vivo CRISPR: Researchers are developing methods to deliver CRISPR-Cas9 directly into the body, enabling in vivo gene editing. This approach has shown promise in preclinical studies for the treatment of liver diseases, muscular dystrophy, and neurodegenerative disorders.
Challenges and Future Directions
Despite the remarkable progress, gene therapy faces several challenges, including:
Delivery Efficiency: Ensuring the efficient delivery of therapeutic genes to target cells remains a major hurdle. Researchers are exploring novel delivery systems, such as engineered viruses and nanoparticles, to improve targeting and reduce off-target effects.
Immune Response: The immune system can recognize and attack viral vectors or genetically modified cells, limiting the effectiveness of gene therapy. Strategies to mitigate immune responses include the use of immune-suppressive drugs and the development of less immunogenic vectors.
Cost and Accessibility: Gene therapies are often expensive, with some treatments costing millions of dollars. Efforts are underway to reduce costs and improve accessibility, particularly in low- and middle-income countries.
India-Specific Developments in Gene Therapy
India has made significant strides in the field of gene therapy, driven by its strong biotechnology sector, academic research institutions, and government initiatives. Some of the key developments include:
Research and Development: Indian researchers are actively involved in gene therapy research, focusing on diseases prevalent in the Indian population, such as thalassemia, sickle cell anemia, and certain types of cancer. Institutions like the Indian Institute of Science (IISc), Tata Institute of Fundamental Research (TIFR), and Centre for Cellular and Molecular Biology (CCMB) are at the forefront of this research.
Clinical Trials: India has conducted several clinical trials for gene therapies, particularly for blood disorders and cancer. For example, researchers at Christian Medical College (CMC) Vellore have conducted trials for gene therapy in beta-thalassemia patients.
Regulatory Framework: The Indian Council of Medical Research (ICMR) and the Department of Biotechnology (DBT) have established guidelines for gene therapy research and clinical trials. These guidelines ensure that gene therapy is conducted ethically and safely, with proper oversight and monitoring.
Collaborations and Partnerships: Indian researchers and institutions are collaborating with international organizations and pharmaceutical companies to advance gene therapy. For example, the Biotechnology Industry Research Assistance Council (BIRAC) has partnered with global organizations to support gene therapy research and development.
Affordable Gene Therapies: India is working towards developing affordable gene therapies to ensure accessibility for its large population. Efforts include the development of cost-effective viral vectors and the establishment of manufacturing facilities for gene therapy products.
Ethical and Social Considerations
Gene therapy raises several ethical and social issues, including:
Informed Consent: Patients must be fully informed about the risks and benefits of gene therapy, particularly in the case of experimental treatments. Ensuring informed consent is crucial, especially in vulnerable populations.
Equity and Access: The high cost of gene therapies raises concerns about equity and access. Policymakers must address these issues to ensure that gene therapies are accessible to all, regardless of socioeconomic status.
Germline Editing: The potential for germline gene therapy to create heritable changes in the human genome raises significant ethical concerns. International consensus is needed to establish guidelines for the responsible use of germline editing technologies.
Public Perception: Public understanding and acceptance of gene therapy are critical for its successful implementation. Efforts to educate the public about the science, benefits, and risks of gene therapy are essential.
Conclusion
Gene therapy represents a transformative approach to medicine, offering the potential to treat, cure, or prevent a wide range of diseases. From its mechanisms and types to its advancements and challenges, gene therapy is a complex and rapidly evolving field. For UPSC aspirants, understanding the science, applications, and ethical considerations of gene therapy is essential, as it intersects with biotechnology, public health, and policy-making.
